- The electrons the neon atoms lose (small blue dots) are negatively charged, so they hurtle the opposite way toward the positive terminal at the other end of the tube. In all this rushing about, atoms, ions, and electrons are constantly colliding with one another.
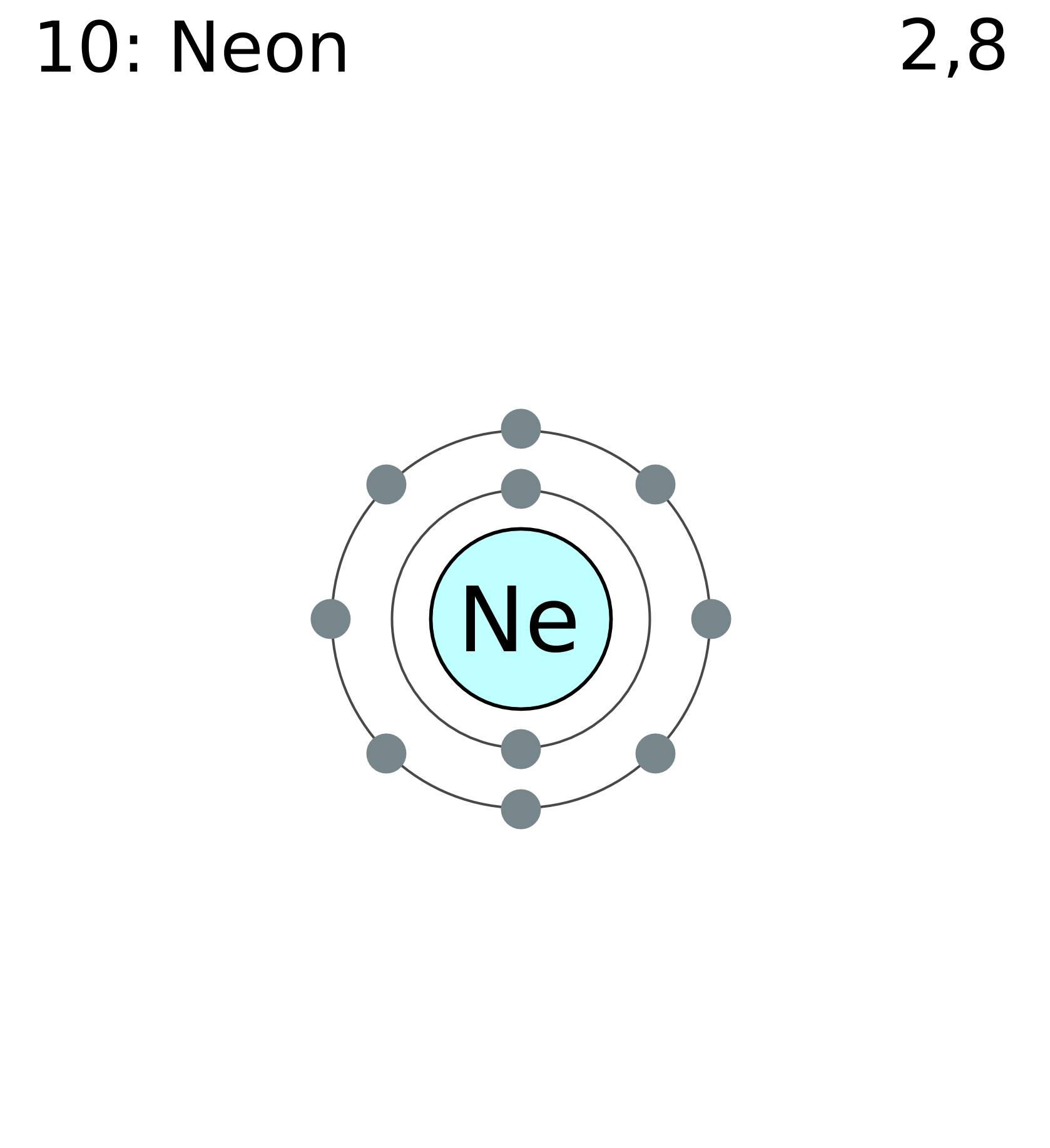
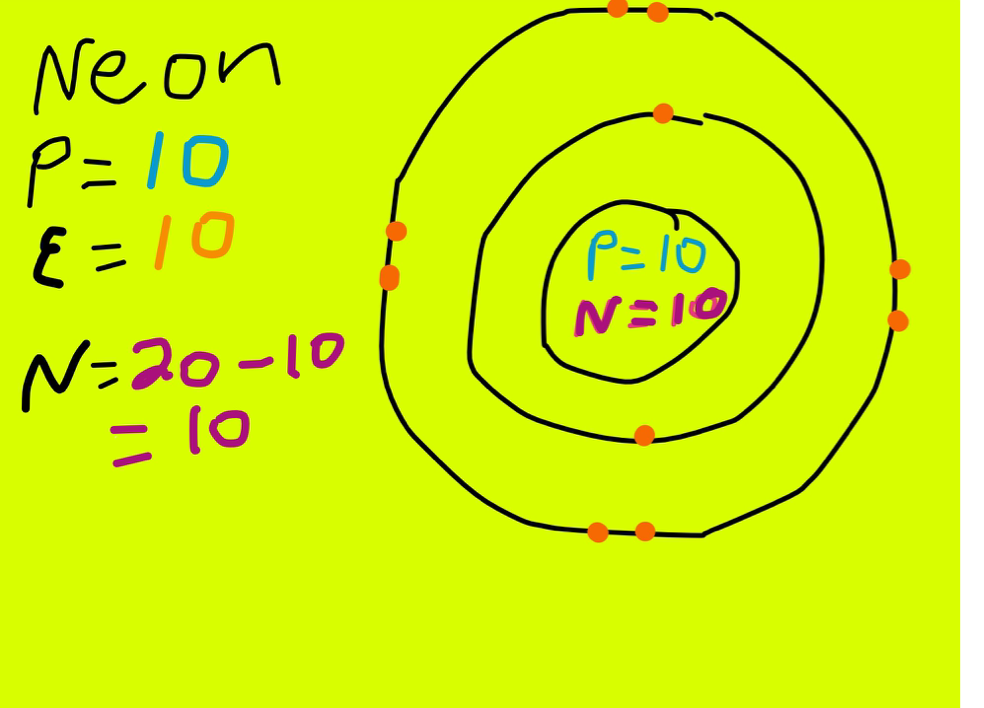
- The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Neon is 10. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z.
- If we look at their atomic numbers (eg from a periodic table) we see that potassium is at 19, and neon at 10. The atomic number equates to the number of protons which in an atom are equal to the number of electrons. Therefore potassium has (19 - 1.
 hat makes night-time cities fizz and crackle with life? Brightly colored
hat makes night-time cities fizz and crackle with life? Brightly colored How many unpaired electrons are in the neon atom? How many unpaired electrons are in the calcium atom? This atom is This atom is A. How many unpaired electrons are in the neon atom?
 neon lamps play a huge part. If you've ever seen the lightsdancing in Tokyo, New York City, or London, you'll knowexactly what I mean. Whole streets seem to leap alive the minutethe neon switches on. Strictly speaking, lamps filled with neon gascan make only red light and you need other gases to make othercolors. In fact, by mixing different gases, it's possible to makeover 150 different colors of 'neon' light—and paint the nightsky with almost any color you like! Let's take a closer look at howthese things work.
neon lamps play a huge part. If you've ever seen the lightsdancing in Tokyo, New York City, or London, you'll knowexactly what I mean. Whole streets seem to leap alive the minutethe neon switches on. Strictly speaking, lamps filled with neon gascan make only red light and you need other gases to make othercolors. In fact, by mixing different gases, it's possible to makeover 150 different colors of 'neon' light—and paint the nightsky with almost any color you like! Let's take a closer look at howthese things work.Example 1 What is the Lewis electron dot diagram for each element? Since there are 10 electrons, 2 go in the first shell but the rest 8 electrons are in the second shell, therefore, these electrons are considered to be in the valence shell, and we label them. When doubling up electrons, make sure that a side has no more than two electrons.
Ionic and Metallic Bonding
We can mark these electrons and indicate what happens to them when an element reacts. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are paired.
However, conventionally, we draw the dots for the two p electrons on different sides. Determine the number of valence electrons for each atom in the molecule 2. A beryllium atom, with two valence electrons, would have the electron dot diagram below. The third electron will go on another side of the symbol: Again, it does not matter on which sides of the symbol the electron dots are positioned.
Ionic and Metallic Bonding
Video: Neon Electron Configuration Notation The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Diagrams contain a lot of useful information in a compact format. What does the diagram above tell us? It is therefore a Nobel Gas. So it would have three dots around the symbol for aluminum, two of them paired to represent the 3s electrons: The valence electron configuration for selenium is 4s24p4. The football play diagrammed above describes the lineup of each player on the team and describes how they will move when the ball is snapped. But in this case, we are drawing electronic dot diagrams. For carbon, there are four valence electrons, two in the 2s subshell and two in the 2p subshell.
The eight valence electrons, a full outer s and p sublevel, give the noble gases their special stability. Because the second energy level 2s22p6 has eight electrons Neon has an octet and has a full outer shell. Its valence electron shell is 2s22p1, so it has three valence electrons. For example, the electron dot diagram for iron valence shell configuration 4s23d6 is as follows: Elements in the same column of the periodic table have similar Lewis electron dot diagrams because they have the same valence shell electron configuration. Electron dot diagrams for ions are the same as for atoms, except that some electrons have been removed for cations, while some electrons have been added for anions. It does not matter what order the positions are used. For neon, we must determine a number of valence electrions b place dots around the element to represent the valence electrons.
This makes it easier to understand and predict how atoms will interact to form chemical bonds. The number of valence electrons can be easily determined from the electron configuration. A blog dedicated for the love and learning of Chemistry. The next atom, lithium, has an electron configuration of 1s22s1, so it has only one electron in its valence shell. Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom.
Diagrams of electrons give similar information about where certain electrons are. All rights reserved. How do we show electrons in atoms? These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. Thus the electron dot diagrams for the first column of elements are as follows: Monatomic ions are atoms that have either lost for cations or gained for anions electrons. Read my article in Science Education based on my dissertation. The remaining six electrons will go in the 2p orbital. This periodic table can also help you when drawing Electronic Dot Diagrams. How to Write the Electron Configuration for Neon Neon is the tenth element with a total of 10 electrons.
Several examples from the second period elements are shown in the Table below. Its electron dot diagram resembles that of hydrogen, except the symbol for lithium is used: Beryllium has two valence electrons in its 2s shell, so its electron dot diagram is like that of helium: The next atom is boron. In writing the electron configuration for neon the first two electrons will go in the 1s orbital. As one proceeds left to right across a period, the number of valence electrons increases by one. When examining chemical bonding, it is necessary to keep track of the valence electrons of each atom. Draw electron dot diagrams for elements. Therefore the Ne electron configuration will be 1s22s22p6.
Compound: We have just learned how to draw a Lewis dot diagram for a single element and a compound. As such, the electron dot diagram for carbon is as follows: With N, which has three p electrons, we put a single dot on each of the three remaining sides: For oxygen, which has four p electrons, we now have to start doubling up on the dots on one other side of the symbol. In the p block, the number of valence electrons is equal to the group number minus ten. As usual, we will draw two dots together on one side, to represent the 2s electrons. The number of dots equals the number of valence electrons in the atom. By going through the periodic table, we see that the Lewis electron dot diagrams of atoms will never have more than eight dots around the atomic symbol.
Lewis Diagrams for compounds and Ions: -In covalent compounds electrons are shared 1.
How to Write the Electron Configuration for Neon
Electron Dot Diagrams Recall that the valence electrons of an atom are the electrons located in the highest occupied principal energy level. For example, the Lewis electron dot diagram for hydrogen is simply Because the side is not important, the Lewis electron dot diagram could also be drawn as follows: The electron dot diagram for helium, with two valence electrons, is as follows: By putting the two electrons together on the same side, we emphasize the fact that these two electrons are both in the 1s subshell; this is the common convention we will adopt, although there will be exceptions later. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms.
Neon Electron Level Arrangement
To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. In making cations, electrons are first lost from the highest numbered shell, not necessarily the last subshell filled. Thus we have Anions have extra electrons when compared to the original atom. Since 1s can only hold two electrons the next 2 electrons for Ne go in the 2s orbital. Place atoms so that valence electrons are shared to fill each orbital.
Neon Electrons Per Shell
In the s block, Group 1 elements have one valence electron, while Group 2 elements have two valence electrons. Post by Ren Flores. A Lewis electron dot diagram A representation of the valence electrons of an atom that uses dots around the symbol of the element. Group 13 has three valence electrons, Group 14 has four, up through Group 18 with eight. Its electron dot diagram is as follows: Test Yourself What is the Lewis electron dot diagram for each element? Describe the electron dot diagram system of representing structure. Valence electrons are primarily responsible for the chemical properties of elements.
