- Name: Phosphorus Symbol: P Atomic Number: 15 Atomic Mass: 30.97376 amu Melting Point: 44.1 °C (317.25 K, 111.38 °F) Boiling Point: 280.0 °C (553.15 K, 536.0 °F) Number of Protons/Electrons: 15 Number of Neutrons: 16 Classification: Non-metal Crystal Structure: Monoclinic Density @ 293 K: 1.82 g/cm 3 Color: white Atomic Structure.
- Phosphorus has an atomic number of 15 so what will be the distribution of its electrons? The first energy level will have 8 and the second will have seven b. The first energy level will have 2, the second will have 8, and the third will have 5. The first energy level will have 2 and the second will have 13. The first, second, and third will have five electrons e. Electron arrangement.
- Phosphorus has an electron configuration of Ne3s2 3p3, the outer most shell has 5 electrons. To gain the electron configuration of a noble gas, 3 electrons are shared with electrons of other atoms.
How many electrons are in an atom of phosphorus?
Phophorus is in group 15, it has 5 electrons in its outer shell, 3s23p3. When it forms chemical compounds it can share electrons to form covalent bonds or gain 3 electrons to form the P3.
Does phosphorus have 15 electrons?
So for the element of PHOSPHORUS, you already know that the atomic number tells you the number of electrons. That means there are 15 electrons in a Phosphorus atom. Looking at the picture, you can see there are two electrons in shell one, eight in shell two, and five in shell three.
Can there be more than 8 electrons in a shell?

It is commonly used to determine the Lewis structure of molecules. However, the octet rule is not the end of the story. The main exception is the family of hypervalent molecules, in which a main group element nominally has more than 8 electrons in its valence shell.
Phosphorus Number Of Valence Electrons
How many electrons does phosphorus have in its valence shell?
The highest-numbered shell is the third shell, which has 2 electrons in the 3s subshell and 3 electrons in the 3p subshell. That gives a total of 5 electrons, so neutral phosphorus atoms have 5 valence electrons.
How many electrons are in the first energy level of phosphorus?
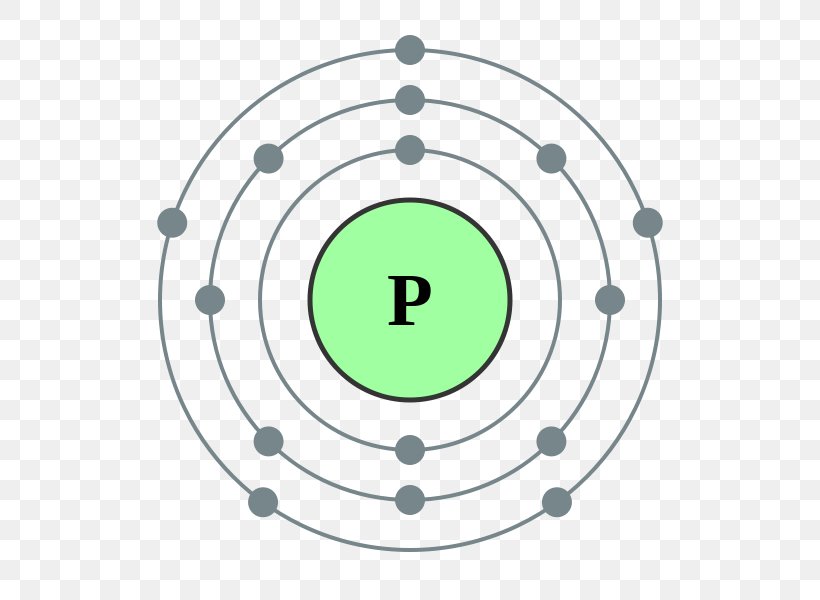
there are 2 electrons at the first energy level, and 8 at the second energy level.
How many electrons are present in a phosphorus 3+ atom?
P (Phosphorus) has an atomic number of 15. Therefore, it has 15 protons. A charge of -3 indicates that it has gained 3 electrons. Therefore, it has 18 electrons.
How are 15 electrons arranged in a phosphorus atom?
When we write the configuration we’ll put all 15 electrons in orbitals around the nucleus of the Phosphorus atom. In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital.
How does phosphorus become stable?
Because of the relatively weak intermolecular attractions (van der Waals forces) between the separate P4 molecules, the solid melts easily at 44.1 °C (111.4 °F) and boils at about 280 °C (536 °F). This may prove to be the most stable form of phosphorus, despite the relative difficulty in its preparation.
What element has 15 protons and 15 neutrons?
| Name | Phosphorus |
|---|---|
| Atomic Mass | 30.974 atomic mass units |
| Number of Protons | 15 |
| Number of Neutrons | 16 |
| Number of Electrons | 15 |
Why is 3rd shell 8 or 18?
In this sense the third shell can hold 8 electrons. In this sense the third shell can hold a total of 18 electrons. So the third shell can be considered to hold 8 or 18 electrons but in total the third shell can hold 18 electrons.
Phosphorus Number Of Protons Neutrons Electrons
Can hold up to 2 electrons?
The general formula is that the nth shell can in principle hold up to 2 (n2) electrons. Each shell can contain only a fixed number of electrons: The first shell can hold up to two electrons, the second shell can hold up to eight ( 2 + 6) electrons, the third shell can hold up to 18 ( 2 + 6 + 10) and so on.
Can phosphorus have more than 8 valence electrons?
Unlike atoms from periods one and two that only have the s and p orbitals (total of 8 valence electrons ), atoms like phosphorus, sulfur, and chlorine can have more than 8 electrons because they are not restricted to the s and p orbitals and have a d orbital for additional electrons needed for bonding.
Why does phosphorus have a valence of 5?
The valency of 5 is due to exitation of an electron from 3s orbitals to 3d orbital. It contains empty 3d orbitals so phosphorus can expand its octet by exciting on electron from 3s to 3d. Hence phosphorus can show valency 3 and 5 both.
Why is the Valency of phosphorus 3 and 5 both?
Phosphorous shows both the valencies 3 and 5. Phosphorous with valency 3: With three unpaired electrons in three p orbitals, phosphorous shows valency 3, like in case of compound PCl3. Thus, phosphorous atom undergoes sp 3 d hybridisation to form five equivalent sp3d orbitals each containing one unpaired electron.
How many electrons are in the outermost shell of phosphorus?
According to the periodic table above, phosphorus belongs to Group 5A. Therefore, Its valence electrons should be 5. The outermost orbitals, 3s2 3p3, contains 5 electrons. Thus, valence electrons for P is 5.
Learning Objectives
- To describe how electrons are grouped within atoms.
Although we have discussed the general arrangement of subatomic particles in atoms, we have said little about how electrons occupy the space about the nucleus. Do they move around the nucleus at random, or do they exist in some ordered arrangement?
The modern theory of electron behavior is called quantum mechanics. It makes the following statements about electrons in atoms:
- Electrons in atoms can have only certain specific energies. We say that the energies of the electrons are quantized.
- Electrons are organized according to their energies into sets called shells. Generally the higher the energy of a shell, the farther it is (on average) from the nucleus. Shells do not have specific, fixed distances from the nucleus, but an electron in a higher-energy shell will spend more time farther from the nucleus than does an electron in a lower-energy shell.
- Shells are further divided into subsets of electrons called subshells. The first shell has only one subshell, the second shell has two subshells, the third shell has three subshells, and so on. The subshells of each shell are labeled, in order, with the letters s, p, d, and f. Thus, the first shell has only an s subshell, the second shell has an s and a p subshell, the third shell has s, p, and d subshells, and so forth.
- Different subshells hold a different maximum number of electrons. Any s subshell can hold up to 2 electrons; p, 6; d, 10; and f, 14.
It is the arrangement of electrons into shells and subshells that most concerns us here, so we will focus on that.
We use numbers to indicate which shell an electron is in. The first shell, closest to the nucleus and with the lowest-energy electrons, is shell 1. This first shell has only one subshell, which is labeled s and can hold a maximum of 2 electrons. We combine the shell and subshell labels when referring to the organization of electrons about a nucleus and use a superscript to indicate how many electrons are in a subshell. Thus, because a hydrogen atom has its single electron in the s subshell of the first shell, we use 1s1 to describe the electronic structure of hydrogen. This structure is called an electron configuration. Electron configurations are shorthand descriptions of the arrangements of electrons in atoms. The electron configuration of a hydrogen atom is spoken out loud as “one-ess-one.”
Phosphorus Number Of Valence Electrons
Helium atoms have 2 electrons. Both electrons fit into the 1s subshell because s subshells can hold up to 2 electrons; therefore, the electron configuration for helium atoms is 1s2 (spoken as “one-ess-two”).

The 1s subshell cannot hold 3 electrons (because an s subshell can hold a maximum of 2 electrons), so the electron configuration for a lithium atom cannot be 1s3. Two of the lithium electrons can fit into the 1s subshell, but the third electron must go into the second shell. The second shell has two subshells, s and p, which fill with electrons in that order. The 2s subshell holds a maximum of 2 electrons, and the 2p subshell holds a maximum of 6 electrons. Because lithium’s final electron goes into the 2s subshell, we write the electron configuration of a lithium atom as 1s22s1.
The next largest atom, beryllium, has 4 electrons, so its electron configuration is 1s22s2. Now that the 2s subshell is filled, electrons in larger atoms start filling the 2p subshell. Thus, the electron configurations for the next six atoms are as follows:
- B: 1s22s22p1
- C: 1s22s22p2
- N: 1s22s22p3
- O: 1s22s22p4
- F: 1s22s22p5
- Ne: 1s22s22p6
With neon, the 2p subshell is completely filled. Because the second shell has only two subshells, atoms with more electrons now must begin the third shell. The third shell has three subshells, labeled s, p, and d. The d subshell can hold a maximum of 10 electrons. The first two subshells of the third shell are filled in order—for example, the electron configuration of aluminum, with 13 electrons, is 1s22s22p63s23p1. However, a curious thing happens after the 3p subshell is filled: the 4s subshell begins to fill before the 3d subshell does. In fact, the exact ordering of subshells becomes more complicated at this point (after argon, with its 18 electrons), so we will not consider the electron configurations of larger atoms.
A fourth subshell, the f subshell, is needed to complete the electron configurations for all elements. An f subshell can hold up to 14 electrons.
Example (PageIndex{1}): Electronic Configuration of Phosphorus Atoms
What is the electron configuration of a neutral phosphorus atom?
Solution

Phosphorus Amount Of Electrons
A neutral phosphorus atom has 15 electrons. Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. That leaves 5 electrons. Of those 5 electrons, 2 can go into the 3s subshell, and the remaining 3 electrons can go into the 3p subshell. Thus, the electron configuration of neutral phosphorus atoms is 1s22s22p63s23p3.
Exercise (PageIndex{1}): Electronic Configuration of Chlorine Atoms
What is the electron configuration of a neutral chlorine atom?
Chemistry results from interactions between the outermost shells of electrons on different atoms. Thus, it is convenient to separate electrons into two groups. Valence shell electrons (or, more simply, the valence electrons) are the electrons in the highest-numbered shell, or valence shell, while core electrons are the electrons in lower-numbered shells. We can see from the electron configuration of a carbon atom—1s22s22p2—that it has 4 valence electrons (2s22p2) and 2 core electrons (1s2).
Example (PageIndex{2}): Counting Valence Electrons in Phosphorus Atoms
From the electron configuration of neutral phosphorus atoms in Example (PageIndex{1}), how many valence electrons and how many core electrons does a neutral phosphorus atom have?
Solution
The highest-numbered shell is the third shell, which has 2 electrons in the 3s subshell and 3 electrons in the 3p subshell. That gives a total of 5 electrons, so neutral phosphorus atoms have 5 valence electrons. The 10 remaining electrons, from the first and second shells, are core electrons.
Exercise (PageIndex{2}): Counting Valence Electrons in Chlorine Atoms
From the electron configuration of neutral chlorine atoms (Exercise (PageIndex{1})), how many valence electrons and how many core electrons does a neutral chlorine atom have?
Concept Review Exercises
- How are electrons organized in atoms?
- What information does an electron configuration convey?
- What is the difference between core electrons and valence electrons?

Answers
Phosphorus P Number Of Electron
- Electrons are organized into shells and subshells around nuclei.
- The electron configuration states the arrangement of electrons in shells and subshells.
- Valence electrons are in the highest-numbered shell; all other electrons are core electrons.
Key Takeaway
- Electrons are organized into shells and subshells about the nucleus of an atom.
